Kuvat / Äänet
Mitä
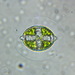
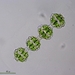
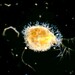
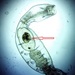
Stentor pyriformisHavainnoija
peptolabKuvaus
Stentor pyriformis Johnson 1893 from the superficial benthos of the river edge of the freshwater segment of estuarine river Peconic River. Imaged in Nomarski DIC on Olympus BH2S using SPlan 10 NA 0.30, SPlanapo 20 NA 0.70, SPlanapo 40 NA 0.95 and SPlan 100 NA1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells were visible with the naked eye accumulating on the surface of the water at the side of the sample container. Cells measure from 650-800 um when fully extended. The cytoplasm is densely filled with green algal zoochlorella symbionts. There are also much smaller colorless cortical and cytoplasmic granules. The dense population of zoochorellae obscured the internal morphology of the stentors. Macronuclei could only be observed in markedly squashed cells by evaporation of water from under the coverglass. Most cells had two spherical macronuclei, occasionally three. The morphology of my population was identical to that described by Hoshina et al 2021, however their population was smaller than mine at 220-500 um and their macronuclear count also differed from my population which showed 2 MA: "The average number of macronuclei was 6.1 (range 4–10, n = 9) for freshly obtained samples, whereas four-year cultured cells contained only one or two" (1).
"S. pyriformis is a poorly described species, although S. pyriformis is clearly distinguishable from other Stentor species. The species was first described in 1893 and then appeared in a microbiota report in 1908. However, its next appearance was not until 1994, in the study on revision of the genus by Foissner and Wolfl. As described in the original literature, difficulties in the cultivation of this species may have hindered the research on this species. In Japan, S. pyriformis can be found only in highland highly oligotrophic moors, suggesting that intracellular symbiotic algae would help this species of Stentor survive in such a harsh environment. S. pyriformis was described by Johnson in 18936. This algae-bearing Stentor has separated spherical macronuclei without pigmentation, which certainly differentiates it from other Stentor species. While the most common algae-bearing Stentor, S. polymorphus assumes a slender trumpet shape (often shortened), S. pyriformis never resembles such a slender trumpet, but assumes a pear or short conical shape, even when it is swimming. Among algae-bearing Stentor spp., S. polymorphus and S. pyriformis only are considered colorless species, whereas colored species are S. amethystinus, S. fuliginosus, S. araucanus, and S. tartari. Therefore, S. pyriformis is a clearly discernible species; however, it remains underexplored" (1).
"Cells of S. pyriformis were broadly trumpet-shaped, usually 220–500 × 120–300 µm. This length–width ratio did not change significantly between the cells attached to something and swimming. The cells were colored green due to their endosymbiotic green algae that were distributed along the whole body. A large number of tiny transparent vesicles were present along the ciliary rows immediately under the cell surface. To see the contents, the crushed cells were observed. Symbiotic algae appeared to be typical Chlorella-like algae, but no dividing alga was observed. The algal cells appeared more vividly green when compared to those in P. bursaria, suggesting that they are richer in photosynthetic pigments. The symbiotic algae in S. pyriformis had the same size and morphology as those in P. bursaria. Macronuclei were, in general, large and spherical (ø 20–35 µm). The average number of macronuclei was 6.1 (range 4–10, n = 9) for freshly obtained samples, whereas four-year cultured cells contained only one or two. Micronuclei could not be identified" (1).
My population had two macronuclei rarely three in contrast to the populations of Hoshina et al which had an average of 6.1 macronuclei (range 4-10). Also, Hoshina et al 2021 (1) did not identify micronuclei in their multimodality study of S. pyriformis which included electron microscopy. However, the study of Walker 1908 (2) describes the presence of two macronuclei with multiple small micronuclei scattered within the macronucleus. I detected similar small nodules within the macronuclei to those depicted by Walker 1908 but I am uncertain if these are nucleoli or micronuclei. Hishina et al 2021 describe " a large number of transparent vesicles were present along the ciliary rows immediately under the cell surface (1) which I also found. In addition, Hoshina et al 2021 describe numerous starch granules in the cytoplasm which I believe are also present in my samples. These granules are more than twice the size of the subcortical vesicles.
- Characterization of a green Stentor with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm. Ryo Hoshina, Yuuji Tsukii, Terue Harumoto & Toshinobu Suzaki. Scientific Reports | (2021) 11:2865 | https://doi.org/10.1038/s41598-021-82416-9
- Observations on the Micro-Fauna of an Oregon Pond. Elda R. Walker. Transactions of the American Microscopical Society, Vol. 28, Twenty-Ninth Annual Meeting (Sep., 1908), pp. 75-84
Kuvat / Äänet
Mitä
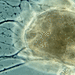
Scaeva affinisHavainnoija
donnalucasKuvaus
Several of these larvae were found on aphid-infested roses. The third image is of a captured one eating an aphid.
Havainnoija
mnold1Kuvaus
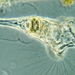
Mag. 400x
- A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
Stalked, pennate diatom. Compare to a similar observation from a different, local waterbody, https://www.inaturalist.org/observations/86792792. It may be that the diatoms in the current observation are all presenting in girdle view... Diatoms from the earlier observation have distinctive profiles in girdle and valve views. The distinction can not be made for the current specimens.
Mitä
Rataseläimet (Pääjakso Rotifera)Havainnoija
mnold1Kuvaus
Mag. 400x
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Possibly a rotifer resting egg.
A similar specimen (in size and configuration) was observed from the same location during Fall 2022 https://www.inaturalist.org/observations/188170793. @shanesmicrope shared an image from Plingfactory that that was very similar to the current and previous specimens. The similarity is with the resting egg of Polyarthra vulgaris https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/E-TL/Bdelloid/Rotifer%20reproduction.html. My suspicion is that my specimens are also rotifer eggs.
Kuvat / Äänet
Mitä
Sienet (Kunta Fungi)Havainnoija
thomasirvineKuvaus
Growing on Coccoloba uvifera
Kuvat / Äänet
Mitä
Suku ThuricolaHavainnoija
mnold1Kuvaus
Mag. 200x (1,4); 400X (2,3)
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Thuricola, a loricate peritrich ciliate. Lorica has a valve flap that must be pushed open to allow the zooids to feed. The lorica is round-bottomed and stalkless. Images in the video, https://youtu.be/om-CaI8zPlk, suggest there may be a vertical indentation at the aperture of the lorica.
Kuvat / Äänet
Mitä
Entomoneis ornataHavainnoija
closterium_mysteriumKuvaus
A water sample was taken from the bank of the Vuoksi River. The sample was stored at room temperature and observed one day after collection.
Video: https://youtu.be/eGHWgdZYq3Q
Observation of Surirella librile https://www.inaturalist.org/observations/192091414
Kuvat / Äänet
Mitä
Suku EucyclopsHavainnoija
dgborinKuvaus
Sample take on 2023-11-29.
Female with eggs.
Kuvat / Äänet
Mitä
Suku HalteriaHavainnoija
dgborinKuvaus
Inside a wet moss sample taken some weeks ago.
Kuvat / Äänet
Havainnoija
closterium_mysteriumKuvaus
A water sample was taken from the shore of Srednerogatsky Pond. The air temperature was 14°C (57.2 °F). The sample was stored at room temperature and observed 4 days after collection.
Video: https://youtu.be/-mJGj69-4JQ
Kuvat / Äänet
Mitä
Purppuraturpiaali (Quiscalus quiscula)Havainnoija
mnold1Kuvaus
Rowdy group of juveniles. Some had a small tuft of white feathers on their wing, highlighted by a white arrow in the following images. A similar tuft is seen in this image of a juvenile https://ebird.org/species/comgra/. I do not know the significance of this tuft... is it a remnant of hatchling plumage?... is it sex specific?...perhaps it is a common feature in all maturing Common Grackles? Interesting!
Kuvat / Äänet
Mitä
Pronssirusokas (Entoloma formosum)Havainnoija
ikhomKuvaus
Growing in a mossy ground.
With angular spores, measured
*(9.6) 9.8 - 11.2 (11.3) × (6.5) 6.8 - 8.1 (8.3) µm
Q = (1.2) 1.3 - 1.5 (1.7) ; N = 11
Me = 10.4 × 7.3 µm ; Qe = 1.4
Mitä
Asplanchna priodontaHavainnoija
plingfactoryKuvaus
Asplanchna priodonta; spedieren with unknown parasites.
More on this here:
https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Asplanchna%20priodonta.html
Kuvat / Äänet
Mitä
Desmidium coarctatumHavainnoija
mnold1Kuvaus
Mag. 400x
Shares the general shape of the most ornate cells in strands of D. graciliceps and D. grevillei. (http://www.digicodes.info/Desmidium.html) However, the junction between cell seem unusually narrow. Size: each cell measures ~45µ long, 40-45µ wide; cell junctions ~12µ wide.
- periphyton samples were collected by the 2023 Desmid Project team of @karolina et al
Kuvat / Äänet
Havainnoija
mnold1Kuvaus
Mag. 100x (1), 400x (2-4)
Images 3 & 4 highlight spines primarily on the primary lobes. For reference images, see http://www.digicodes.info/Micrasterias_compereana.html#2012018002 and https://www.algaebase.org/search/images/detail/?img_id=19423
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
Kuvat / Äänet
Havainnoija
mnold1Kuvaus
Mag. 400x
Empty semi-cell of desmid Xanthidium scrobiculatum (previously X. cristatum var. scrobiculatum). This taxon is named for the shallow pits (scrobiculae) decorating the face of the semi-cell. In this specimen, 13 scorbiculae form a sideways diamond shape. Two additional scrobiculae are sited within the diamond to the left and right of center. For information and images, see https://botany.natur.cuni.cz/neustupa/stastny-et-al-2013.pdf, p. 405 and Fig. 4.
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
Kuvat / Äänet
Mitä
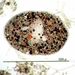
Lahko NassulidaHavainnoija
mnold1Kuvaus
Mag. 100x (1,2,3); 400x (4,5)
...Originally thought this was a flatworm, as indicated by the initial Notes. However, my opinion changed based on the comments of more experienced observers.
Large, multi-color vacuole-filled flatworm (of Suborder Dalytyphloplanida). The outer wall is thick and covered with very fine hair-like processes. Uncertain regarding the ID, especially since I don't see a mouth. For a video, see https://youtu.be/0uItTOfCxN8.
- A water sample was taken on 4/18/2023, from the shore of Avery Swamp impoundment, using a 10µ dip net to enrich for microbes. Air temp. 50°F.
Mitä
Kottarainen (Sturnus vulgaris)Havainnoija
johndreynoldsKuvaus
Clover Island, Kennewick, Washington, USA
Kuvat / Äänet
Mitä
Suku NoctuaHavainnoija
watercoloristKuvaus
Found in damp leaf debris on a chilly day.
Mitä
Viherlevät (Pääjakso Chlorophyta)Havainnoija
mnold1Kuvaus
Mag. 400x
5 independently observed specimens. All are similarly sized and have a relatively thick mucilaginous sheath. @karolina noted that it is unlikely these are individual cells of Hyalotheca since a stellate chloroplast would be readily apparent (e.g. https://www.inaturalist.org/observations/74166151).
- A water sample was taken on 1/24/2023, from the shore of Rosemond Lake , using a 10µ dip net to enrich for microbes. Air temp. 42°F.
Kuvat / Äänet
Mitä
Pediastrum angulosumHavainnoija
mnold1Kuvaus
Mag. 400x
I think the ID is correct.
Interestingly, the empty perimeter cells give a clue regarding how neighboring cells are joined. In the 3rd photo, yellow arrows point to cell junctions that look somewhat like a wood worker's "finger joint". https://www.linkedin.com/pulse/production-tips-finger-joint-edge-glue-panels-nicole-hong. The wavy joint (mimicking interleaved fingers) maximizes the gluing surface area and thereby strengthens the resulting joint. Perhaps these cells are utilizing a similar strategy to strengthen cell contact and therefore colony integrity.
- A water sample was taken on 1/24/2023, from the shore of Rosemond Lake , using a 10µ dip net to enrich for microbes. Air temp. 42°F. The sample was keep at room temp. and re-assayed on 1/31/2023.
Kuvat / Äänet
Mitä
Suku BicosoecaHavainnoija
mnold1Kuvaus
Mag. 400x
Bicosoeca sp., I think. This is a filter-feeding flagellate housed in a transparent, vase-like lorica, and has an anterior flagellum (for feeding and transportation) and an posterior flagellum that anchors to the base of the lorica and allows the cell to retract into the lorica when necessary. These Bicosoeca are living epiphytically on a colony of diatoms, Asterionella formosa.
For 2 videos see: https://youtu.be/GjPdiDE_VOY and https://youtu.be/vCKK5l6yxFM.
For a fabulous a video and photos of Bicosoeca, see the observation recorded by iNatter closterium_mysterium, https://www.inaturalist.org/observations/145952656#activity_comment_7f982c87-a61d-4cd0-88b0-cf67d9969e39.
- A water sample was taken on 1/24/2023, from the shore of Rosemond Lake , using a 10µ dip net to enrich for microbes. Air temp. 42°F.
Mitä
Alaluokka BdelloideaHavainnoija
closterium_mysteriumKuvaus
A water sample was taken from freshwater aquarium. The aquarium had been in room temperature storage.
Just a funny image of a rotifer.
Mitä
Holophrya ovumHavainnoija
plingfactoryKuvaus
Holophtrya-species with symbiotic zoochlorellae. More on this here:
https://www.plingfactory.de/Science/Atlas/KennkartenProtista/01e-protista/e-Ciliata/e-source/Holophrya%20ovum.html
Kuvat / Äänet
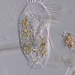
Mitä
Allogromia terricolaHavainnoija
peptolabPaikka
YksityinenKuvaus
Allogromia terricola (Leidy, 1874) syn. Gromia terricola Leidy, 1874 original description), syn. Gromia fluviatilis Dujardin. A terrestrial species of forminefera from an infusion prepared from the soil at the base of my compost heap.
Test spherical to subspherical; smooth or sparsely covered with siliceous particles; yellowish cytoplasm fills the test; aperture not seen; a large nucleus and numerous contractile vacuoles; filopodia long, often enveloping test; on aquatic plants, in moss or soil.
Envelope spheric or subspheric, seldom changing shape; protoplasm habitually covering the surface of the envelop. Pseudopodia numerous, anastomosing. Habitat aquatic plants. Measurements
90-250 um long.
The following discussion is adapted from the reference below.
Foraminifera are unicellular eukaryotes characterized by the presence of granuloreticulopodia and the possession of a membranous, agglutinated, or calcareous test, which is either monothalamous (single-chambered) or polythalamous (multi-chambered) (Loeblich and Tappan 1987). Within monothalamids some species like Reticulomyxa filosa are amoeboid naked forms.
Until 1859, foraminifera were only known from marine habitats, but that year Claparède and Lachmann described a monothalamid foraminifer, Lieberkuehnia wageneri, sampled from an unknown water body in Berlin. It had a smooth flexible test with an entosolenian tube that separated the main cytoplasm mass from the pseudopodial peduncle.
In 1886 Henri Blanc, a Swiss scientist, described another freshwater foraminifer, Gromia brunneri, which he had collected from the bottom of Lake Geneva. This single-chambered species had an agglutinated test, an organic layer covered and/or embedded with foreign, mainly non-organic, particles.
In subsequent years, Eugène Penard, another Swiss protozoologist, described four similar species Gromia gemma and G. squamosa (1899), G. linearis (1902) and G. saxicola (1905) from the same lake. He also described G. nigricans (1902), which he found not far from Lake Geneva in Mategnin and a marsh near Rouelbeau. Penard made permanent preparations of these foraminifera, which are still preserved and available in the Penard Collection of the Natural History Museum of Geneva (Switzerland).
In 1904, Ludwig Rhumbler erected the subfamily Allogromiinae for monothalamous foraminifera characterized by a more or less flexible organic test wall commonly with one or rarely two terminal apertures at either end of the test. He included all described freshwater species in this taxon. In a recent higher ranked classification of foraminifera based on molecular phylogenies (Pawlowski et al. 2013), monothalamous foraminifera were considered as a paraphyletic group that contains agglutinated and organic walled species as well as “naked” amoeboid species and environmental clades with unknown morphological affinities.
Traditionally the organic-walled foraminifera are called allogromiids. Most of them are distributed over a wide range of marine and brackish habitats (Gooday 2002). Freshwater allogromiids with an agglutinated test were originally placed in the genus Gromia by their discoverers, but as its type species G. oviformis is a filose marine species, Rhumbler (1904) transferred three species (G. squamosa, G. nigricans and G. linearis) to Rhynchogromia Rhumbler 1894. He further erected a new genus, Diplogromia, for the other two species having a double test wall: G. brunneri and G. gemma, although without designing a type species for the genus.
De Saedeleer (1934) revised Rhumbler’s classification leaving D. gemma in its genus and creating a new genus Allelogromia for the Rhynchogromia species with G. brunneri as type species. Deflandre (1953) erected the genus Penardogromia for G. linearis, with the argument that it had a homogenous agglutinated test with calcareous particles. Loeblich and Tappan (1960) argued that the classification of De Saedeleer was unacceptable, because G. brunneri had been fixed as the type of Diplogromia by subsequent designation of Cushman (1928). They created the genus Saedeleeria for G. gemma, transferring G. squamosa and G. nigricans also to Diplogromia, but without giving any supporting explanations. Another agglutinated allogromiid, Penardogromia palustris, was described by Thomas (1961) from a freshwater marsh near Bordeaux (France).
Beside these descriptions there have been some scattered records of agglutinated freshwater allogromiids over the years (Grospietsch, 1958, Hoogenraad and De Groot, 1940, Siemensma, 1982, Wailes, 1915; Meisterfeld pers. comm.; Clauss, unpublished) and some photomicrographs available online (Revello, 2015, Protist Information Server, 2016).
Leidy (1879) was the first who described an allogromiid foraminifer, Gromia terricola, from a terrestrial habit. He found this non-agglutinated species “among moist moss in the crevices of pavements, in shaded places, in the city of Philadelphia”. A similar terrestrial organic walled allogromiid Edaphoallogromia australica has been described by Meisterfeld et al. (2001).
Siemensman et al. Taxonomic revision of freshwater foraminifera with the description of two new agglutinated species and genera. European Journal of Protistology. Volume 60, August 2017, Pages 28-44 https://doi.org/10.1016/j.ejop.2017.05.006
Kuvat / Äänet
Mitä
Collotheca ornataHavainnoija
peptolabKuvaus
Collotheca species, possibly C. ornata, attached to the Elodea from fresh water Town Pond. Collotheca belongs to the rotifer class Monogononta, rotifers with only one ovary. These rotifers are sessile; they are either attached to each other forming a spherical colony, or attached individually to the substrate. Each rotifer secretes a gelatinous tube into which it withdraws when disturbed. Phase contrast Zeiss Photomicroscope III using Neofluar PH2 160.40 with Optovar set at x1.6 plus variable phone cropping. At one point, a little Menoidium decided to tempt fate and tease the rotifer by poking its infundibulum.
The absence of setae inserting between the lobes points to Collotheca ornata (thanks @shanesmicroscope ) in contrast to the similar species C. coronetta which has setae on the surfaces between the lobes (see illustration from Shiel and Plewka below).
https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Collotheca%20ornata%20cornuta.html
Collotheca Harring, 1913
Class Rotatoria: Order Paedotrochida: Family Collothecidae
Synonym Floscularia Ehrenberg, 1832
Corona very large, circular, lobed, or pointed. The margins of the corona are furnished with long, very fine setae. Setae not arranged in whorls. The digestive system is very characteristic. The mastax has incudate trophi. Mostly sessile forms, the Collothecans are usually found in clear, gelatinous tubes. In a clear gelatinous tube. Foot terminated by a long, nonretractile peduncle, ending in an adhesive disc. Mostly sessile. Five species are free-swimming and may be found in lake plankton.
Kuvat / Äänet
Havainnoija
lamawebberKuvaus
Thalassiosira pacifica Gran & Angst, 1931
Phylum: Bacillariophycophyta, Subphylum: Bacillariophytina, Class: Mediophyceae, Order: Thalassiosirales, Family: Thalassiosiraceae.
SEM images of the marine diatom Thalassiosira pacifica Gran & Angst, 1931.
Girdle view: Cells 16.1-28.8 µm (7.0–55.0) µm in diameter. GI = n = 3.
Cells rectangular with rounded low mantle. Valve face flat or slightly concave. Connecting thread about as long as the pervalvar axis.
Valve view:
Loculate areolae in linear (straight), eccentric or fasciculate patterns (curved) rows depending on the diameter of the cell. Central process adjacent to a central areola (annulus).Valve face areolae 10-19 in µm (Hasle and Syvertsen 1996, Li et al. 2014). GI = 14-16 in 10 µm. 20 to 28 areolae in 10 µm (Hoppenrath et al. 2007, not so). Valve face covered by tiny siliceous granules (Hoppenrath et al. 2007). Marginal strutted processes (MSPs) with relatively long and coarse external tubes. GI specimens have a bulbous and coarse flared tip. MSPs 4-7 in 10 µm. GI = 6. Separation between MSPs = 1 µm (GI) Mantle areolae smaller than those on valve face. The height of mantle is about 2–3 areolae (Li et al. 2014). Labiate process positioned as for a marginal strutted process, slightly inside the marginal ring. Labiate process slightly larger than the marginal strutted processes.Valve margin ribbed.
Notes: Mantle areolae smaller than those on valve face. Valve margin ribbed. Distinguished from T. angulata by the ribbed margin and the more closely spaced marginal processes with shorter
external tubes and the location of the labiate process. (Hasle and Syvertsen 1996:37, Mahood et al. 1986, Hoppenrath et al. 2007, Li et al. 2013. (Hoppenrath et al. 2007 –Fig. 45-46, scale bar = 10 µm)
Methods:
Collected by a 20 µm plankton net May 30, 2022 from the Spanish Hills Wharf (N48˚ 59.688’, W123˚ 35.064’), Trincomali Channel, Galiano Island, Southern Gulf Islands, British Columbia, Canada. No fixation. A 7.5 mL sub sample of concentrated cells pipetted into a 50 mL poly centrifuge tube. Added 37.5 mL of 30% hydrogen peroxide and mixed. Three days in hydrogen peroxide at room temp, followed by 5 x 45 mL washes of sterile distilled and deionized water. Pipetted and syringed with further washes of sterile distilled and deionized water using a Swinnex filter holder onto 12 mm glass 0.45 µm filters and attached to 13 mm SEM stub with double sided tape Imaging with a Hitachi s4800 and a Hitachi TM4000 SEMs at the AMF at UVIC. Thank you Ron Read for taking some of tthe SEM images and Elaine Humphrey for SEM support. Adjusted in Photoshop.
References:
Cupp, E. E. 1943. Marine Plankton Diatoms of the West Coast of North America. University of California Press. Berkeley, California.
Gran, H.H. and Angst, E.C. (1931). Planktonic Diatoms of Puget Sound. Seattle, University Press. https://babel.hathitrust.org/cgi/pt?id=uc1.31822010334225&view=1up&seq=3
Hoppenrath, M., Beszteri, B., Drebes, G., Halliger, H., Van Beusekom, J.E.E. , Janisch, S. and Wiltshire, K. H. (2007) Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German Bight) and Sylt (North Frisian Wadden Sea) – a first approach to assessing diversity, European Journal of Phycology, 42:3, 271-288, DOI: 10.1080/09670260701352288
Horner, R.A., Postel, J.R., Halsband-Lenk, C., Pierson, J.J., Pohnert, G. and Wichard, T. 2005.Winter-spring phytoplankton blooms in Dabob Bay, Washington. Prog. Oceanogr. 67 (3-4): 286-313.
Hasle, G.R. & Syvertsen, E.E. (1996). Marine Diatoms. In: Identifying Marine Phytoplankton. (Tomas, C.R. Eds). San Diego: Academic Press.
Guiry, M.D. & Guiry, G.M. 2007, AlgaeBase version 4.2. World-wide electronic publication, National University of Ireland, http://algaebase.org, searched April 10, 2022.
Li, Y. and Lu, S. (2013). The genus Thalassiosira off the Guangdong coast, South China Sea. Botanica Marina; 56(1): 83–110. DOI: 10.1515/bot-2011-0045
Mahood, A.D., Fryxell, G.A., & Mcmillan, M. (1986). The diatom genus Thalassiosira: species from the San Francisco Bay system. Proc. Calif. Acad. Sci. 24: 127–156.
Round, F.E., Crawford, R.M. and Mann, D.G. (1990). The Diatoms, Biology & Morphology of the Genera. Cambridge University Press, Cambridge, UK. pp. 132-133.
Hay, M. B., Pienitz, R., and Thomson, R.E. (2003). Distribution of diatom surface sediment assemblages within Effingham Inlet, a temperate fjord on the west coast of Vancouver Island (Canada). , 48(3-4), 291–320. doi:10.1016/s0377-8398(03)00025-2
Sancetta, C. and Calvert S. E. (1988). The annual cycle of sedimentation in Saanich Inlet, British Columbia:implications for the interpretation of diatom fossil assemblages. Deep-Sea Research, Vol. 35, No. 1, pp. 71-90.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC.
Waters, R. E., Brown, L.N., and MG Robinson, M.G. (1992). Phytoplankton of Esquimalt Lagoon, British Columbia: comparison with west Vancouver Island coastal and offshore waters. Canadian Technical Report of Hydrography Ocean Sciences 137.
Kuvat / Äänet
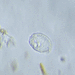
Mitä
Mesodinium pulexHavainnoija
peptolabKuvaus
Mesodinium species, 35-40 um from the shoreline benthos of estuary Hog Creek. The trichites are particularly well-visualized.
Mesodinium Stein, 1862 (ref. ID; 3540, 3690, 4352) or 1863 (ref. ID; 2013, 4613, 4971)
From Dr. Inaki
Class Kinetofragminophora: Subclass Gymnostomata: Order Haptorida: Family Didiniidae (ref. ID; 2013)
Family Mesodiniidae: Litostomatea (ref. ID; 4971)
[ref. ID; 2013]
Body shape pyriform but divided into two sections by a waist-like furrow. Anterior narrows apically, posterior broadly rounded. Oral aperture apical, cytopharynx with 8 to 12 protruding trichites which are forked at the anterior most end. Somatic ciliation reduced to 2 bands of cilia which arise from the central body furrow. Both bands arise from short oblique rows of kinetosomes, the posterior band consists of rows components of the anterior ciliary band are stiff and bristle-like, those of the posterior band are membranelle-like and project backwards. Macronucleus centrally located. Contractile vacuoles have been reported in both posterior and mid-lateral positions.
Kuvat / Äänet
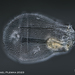
Mitä
Suku ChlamydonellaHavainnoija
peptolabKuvaus
A Chlamydoinella species from the intertidal benthos of marine estuary Accabonac Harbor. The tide was quite low affording access to more bottom than usual. Imaged in Nomarski DIC using Olympus BH2 under SPlan 40x objective and in phase contrast using Zeiss Photomicroscope III using Neofluar PH2 40x objective both with variable phone cropping on Samsung Galaxy S9+.
Chlamydonella
Chlamydonella Deroux, 1970 (ref. ID; 2013)
From: https://www.nies.go.jp/chiiki1/protoz/morpho/ciliopho/chl-ella.htm
Class Kinetofragminophora: Subclass Hypostomata: Order Cyrtophorida: Suborder Chlamydodontina
Ciliophora: Cyrtophorida
Oval in outline shape, dorso-ventrally flattened with dorsal surface strongly arched. Somatic ciliation reduced to central surface with a short field of cilia just on the left of the anterior dorsal surface. Oral aperture supported by basket of trichites. On the right of the ventral surface there are several kineties of which 2 curve around the apex. The extreme right kinety is discontinuous in two parts, one in the equator the other just dorsally on the extreme left of the apex. Below the oral aperture is the left field of kineties (10 or more) which traverse the body width. The arrangement of the pre-oral ciliature is a major characteristic of the genus, the kinety just in front of the oral aperture is always Y-shaped and above it lies a second pre-oral kinety which is simply curved. Macronucleus large, centrally located. There are 2 contractile vacuoles, one anterior, one posterior.
Genus Chlamydonella Deroux in Petz et al. (1995) According to the newly-defined diagnosis (Song and
Wilbert 2000a), the genus Chlamydonella is characterized by: Lynchellids without plasmatic protrusions on ventral side; somatic kineties making no noticeable naked gap between left and right areas; perioral kineties continuous or slightly fragmented with leftmost rows parallel to each other, which are arched transversely; cytopharyngeal rods (nematodesmata) toothed. Macro-
nucleus basically dimorphic.
The Chlamydonella in my isolate measure 50 um in average length (range 45-60 um) and are acontractile but flexible, oval to reniform with an anterior left-hand rostrum, sometimes with a tooth-like ledge. They are dorso-ventrally flattened, ventral surface flat, dorsal surface arched except for an anterior flattened region. Somatic cilia restricted to ventral surface consisting of several longitudinal kineties estimated to number 12.
They feature a poorly visualized central ovoid macronucleus and from one to three contractile vacuoles that are arrayed diagonally usually mid to anterior in position. Ingested meals of small algae and occasionally diatoms are present. The indistinct oval cytostome and a very difficult to discern nematodesmal basket are seen only vaguely in screen capture stills. This inability to visualize the cyrtos in vivo and only faintly in silver preparations has been noted in some species even using differential contrast. I have included several screen captures with the cytostome and/or the cytopharyngeal basket marked with an asterisk.
https://www.researchgate.net/publication/236839421
Redescriptions of three cyrtophorid ciliates from marine biofilm, with
establishment of a new genus, Wilbertella nov gen. (Ciliophora : Cyrtophorida : Lynchellidae). Jun GONG and Weibo SONG. Acta Protozool. (2006) 45: 153 -165
https://www.researchgate.net/publication/236839421
Faunistic Studies on Marine Ciliates from the Antarctic Benthic Area,
Including Descriptions of One Epizoic Form, 6 New Species and, 2 New Genera (Protozoa: Ciliophora). Weibo SONG and Norbert WILBERT. Acta Protozool. (2002) 41: 23 - 61 : https://www.researchgate.net/publication/228590356
Kuvat / Äänet
Mitä
Elämä (Elämä)Havainnoija
mnold1Kuvaus
Mag. 400x
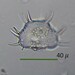
Decorated with distinctive, blunt spines. Small amoeboid test, no pseudopodia visible (Centropyxis)? Pollen grain? Egg? Survival cyst? Small naval mine?
- A water sample was taken on 11/08/2022, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 55°F.
Kuvat / Äänet
Mitä
Pediastrum angulosumHavainnoija
mnold1Kuvaus
Mag. 400x
Colony ~150µ at its widest; cells ~20µ wide. As see here https://bsapubs.onlinelibrary.wiley.com/doi/full/10.1002/ajb2.1066 in Fig. 1.D, 1. F. (Aside: this paper, co-authored by an algal guru here on iNat, suggests that P. angulosum and P. duplex are more similar at the genetic level than their differences in morphology would suggest. Interesting!)
- A water sample was taken on 11/08/2022, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 55°F.
Havainnoija
geraldojprPaikka
Rua Marquês de Paranaguá nº200 - caixa 209 - Centro, Ilhéus - BA, 45653-020, Brasil (Google, OSM)Kuvaus
A Tropical variety of Micrasterias torreyi
Kuvat / Äänet
Mitä
Staurodesmus lobatusHavainnoija
mnold1Kuvaus
Mag. 400x.
Desmid with round semi-cells and large mucilaginous capsule (marked by white lines in right panel of first image). Narrow isthmus with wide open sinuses. I think this is Cosmarium fuelleborniforme, as seen here http://www.digicodes.info/Cosmarium_fuelleborniforme.html. Even though this specimen looks a lot like Sondylosium panduriforme, including the presence of a broad capsule (this latter taxon was also identified in this water sample), but the current specimen is about twice as long and wide as S. panduriforme cells (as seen here https://www.inaturalist.org/observations/132721823)
- A water sample was taken on 08/27/2022 from the shore of Long Pond using a 10µ dip net to enrich for microbes. Air temp. 80°F.